Pharmacovigilance Polaris Beta
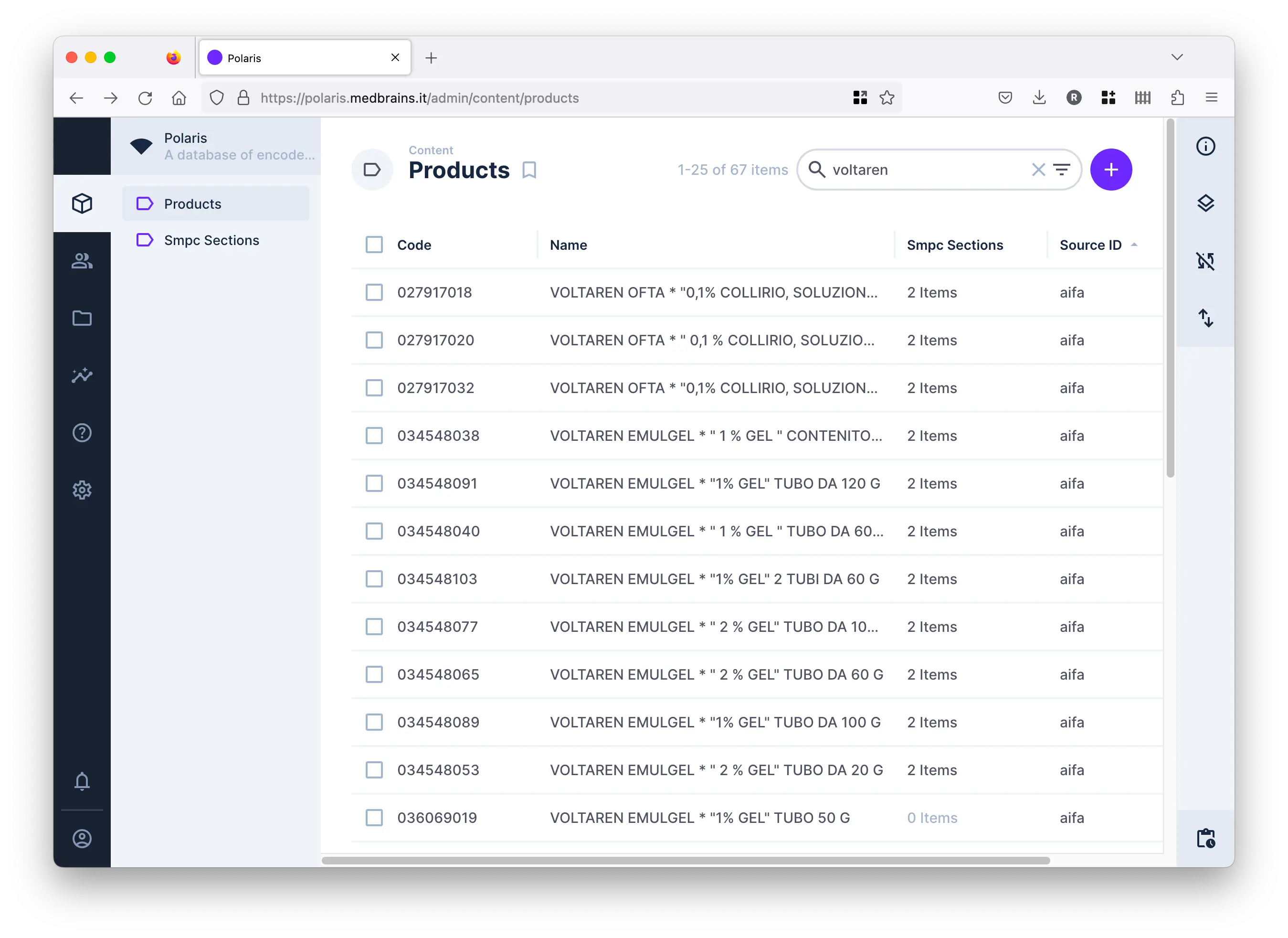
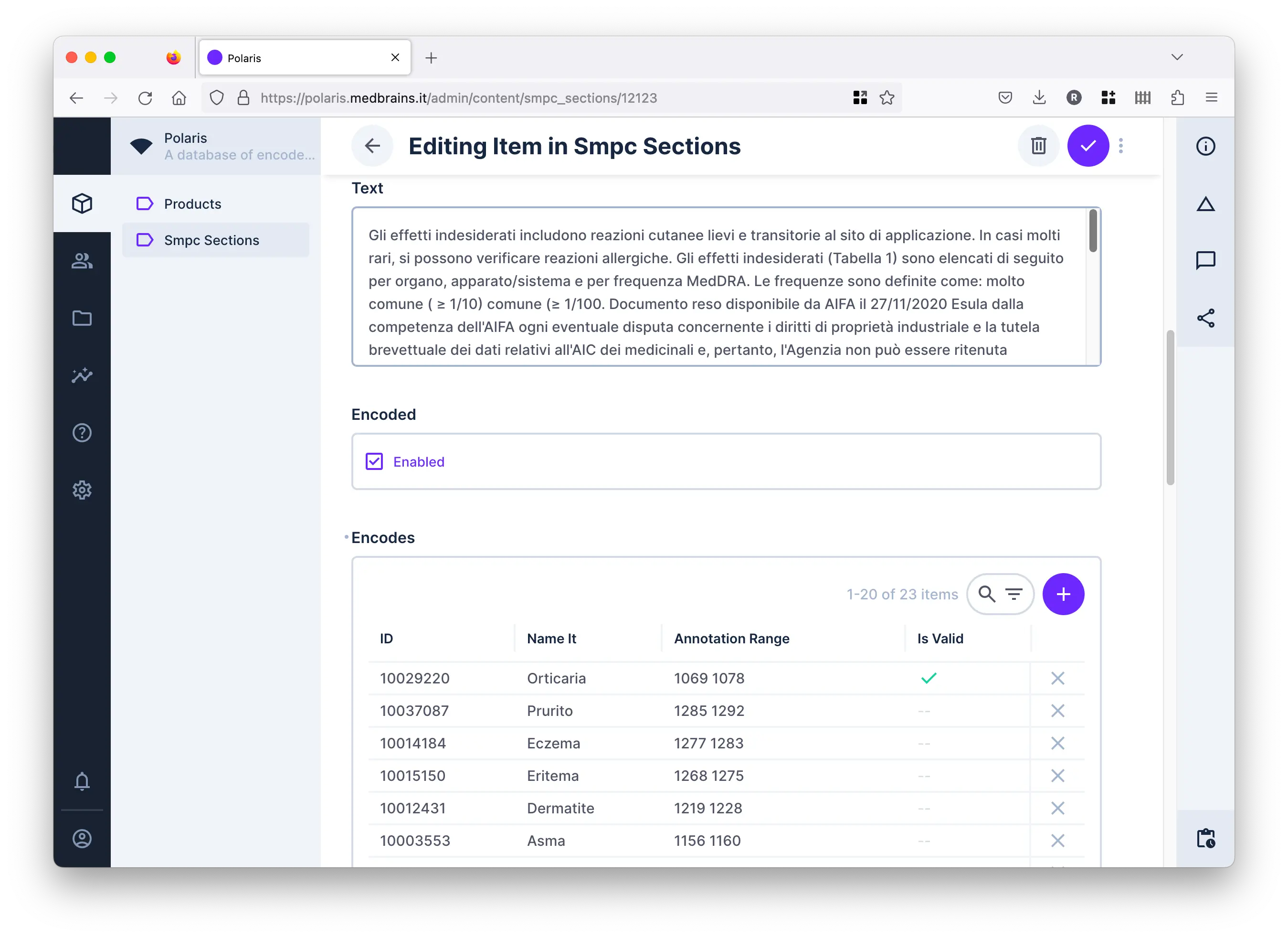
Is a query-able archive of therapeutic indications and adverse reactions reported on Summary of Product Characteristics (SmPCs) encoded as MedDRA Lowest Level Terms (LLTs).
Use cases
Safety report analysis
Check if a reaction is known for a drug. Detect an off-label use of a drug. Sort drug-reaction pairs in signal detection.
SmPCs harmonization
Harmonize SmPCs among medicinal products with the same pharmaceutical composition.
Drug deprescription
Automatically spot medical conditions treated by a drug that could be adverse reactions of another one.
Features
It is currently based on AIFA and EMA SmPC archives, and it could be extended, upon request, to other datasources.
It is automatically built through web-scraping and text auto-coding procedures.
It can be validated manually, upon request, through a dedicated graphic user interface.
It is query-able online, and it can provide aggregated information about similar drugs and at different levels of MedDRA terminology.
Sample dataset
| Data source | Medicinal Product | SmPC data |
|---|---|---|
| AIFA | 022252062 - Zymaflour | Download |
| EMA | EMEA/H/C/004411 - Verkazia | Download |
| EMA | EMEA/H/C/005852 - Amvuttra | Download |